The Search for Pain Relief
It can take time to find a treatment for chronic pain that delivers lasting relief. That search can be frustrating, because pain disrupts everyday life. People with chronic pain often try several different therapies before they find one that provides significant pain relief.

medications
physical
therapy

injections

nerve pressure relief surgery
While these treatments can offer some relief, many people with chronic pain experience less relief with these treatments over time. Fortunately, other treatments for chronic pain can offer lasting relief.
Well-Established Pain Treatment
Even if your chronic pain persists after you’ve tried many types of treatments, it can still be possible to find effective therapy. Talk to the experts at Louisiana Pain Care to see if neurostimulation is right for you. When chronic pain symptoms are under control, you can focus on other things in your life.
Neurostimulation, also known as spinal cord stimulation (SCS) therapy, is a well-established pain treatment used by doctors for more than 50 years.1
How Neurostimulation Works
A neurostimulation system works similarly to a pacemaker. The implantable pulse generator (IPG) is implanted in the body along with thin, insulated wires called leads. When your system is turned on, the generator sends mild electrical pulses through the leads to nerves along your spinal cord to reduce the level of pain you feel.

Neurostimulation interrupts pain signals
When we experience an injury or physical trauma, nerve endings known as pain receptors send a signal along the spinal cord to the brain. The brain interprets that signal as pain.
Spinal cord stimulation, also known as neurostimulation therapy, intercepts these signals so the brain doesn’t receive them. As a result, the person doesn’t experience pain in the same way. For people who live with chronic pain from an injury or condition, neurostimulation may offer relief from chronic pain.
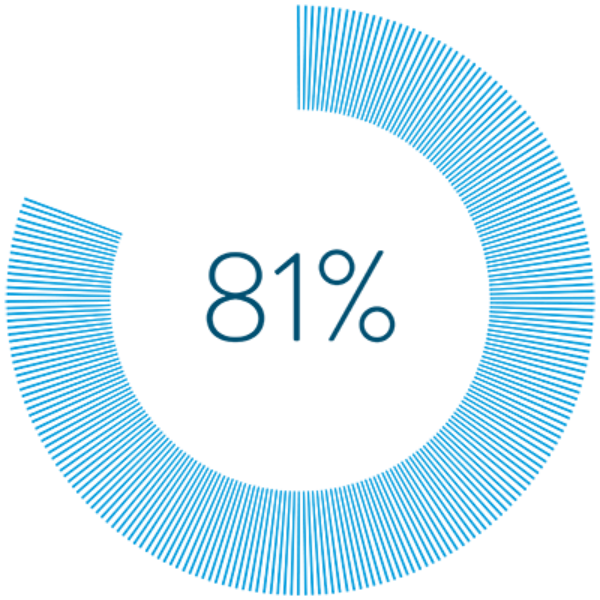
More than 80% of people say they prefer BurstDR™ stimulation, available with SCS therapy from Abbott, to traditional SCS.2
Pain Relief Starts Here
Find the device that is right for you.

Eterna™ SCS System
The Eterna™ SCS System with Xtend™ energy technology reduces charging frequency to five times per year, 3,4 making it the lowest recharge burden platform on the market.5** FlexBurst360™ therapy is the next generation of BurstDR™ stimulation that provides tailored stimulation for up to six areas of pain.6

Proclaim™ Plus and Proclaim™ XR SCS Systems
The Proclaim™ SCS Family offers recharge-free SCS therapy for up to 10 years at lowest-dose settings.***
The Proclaim™ Plus SCS System features FlexBurst360™ therapy, the next generation of clinically proven BurstDR™ stimulation,7 which offers relief for up to six painful areas.6
Be Inspired by People Who Found Relief
Explore the inspiring personal stories of people who’ve reclaimed their lives from chronic pain. Find out how spinal cord stimulation (SCS) or dorsal root ganglion (DRG) therapy can help people with chronic pain live fuller lives – and see firsthand what life is like with an implanted neurostimulator.
“Now I have my life back” – Ann’s Story
“It’s night and day” – Josh’s story
“Feeling free from pain” – Robert’s story
These are actual patient stories. The stories are the experience of these individuals only. Although these patients did not experience complications, there can be risks and potential complications associated with the use of medical devices. If you are interested in learning more, please consult the experts at Louisiana Pain Care.
Talk with us about this Treatment Option
Schedule a consultation with us or ask if this is a treatment option for you at your next appointment. For more information about interventional spine care, please call Louisiana Pain Care, with convenient locations in Monroe and Ruston. Complete this form or request an appointment by calling (318) 323-6405.
* When compared to traditional tonic stimulation.
** Upon implant of the Eterna™ SCS System, approximately three hours five times per year (69 to 74 days between charges) or one hour per month (25 to 27 days between charges) at standard (nominal) settings for BurstDR™ programming: 30/90 dosing when programmed with amplitude of 0.6mA and all other BurstDR™ stimulation settings are left at default. Recommended recharge frequency and duration for competitor product described in their respective IFU or clinical studies, which may involve different patient populations and other variables. Not a head-to-head comparison of stimulation settings or clinical outcomes.
*** Up to 10 years of battery longevity at the lowest dose setting: 0.6mA, 500 Ohms, duty cycle 30s on/360s off. NOTE: In neurostimulation therapy, ‘dose’ refers to the delivery of a quantity of energy to tissue. Safety comparisons and specific dose-response curves for each dosage have not been clinically established. Refer to the IFU for additional information. Hassle-free means recharge-free.
- International Neuromodulation Society. A brief history of neuromodulation. Updated August 3, 2022. Accessed February 14, 2024. https://www.neuromodulation.com/brief-history-neuromodulation
- Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56-66. doi:10.1111/ner.12698
- Abbott. Eterna™ SCS IPG Battery Recharge Characterization Report (90903492). 2023.
- Abbott. Eterna™ SCS IPG Elect Design Verification Report: Current Draw (90860050). 2022.
- Abbott. Eterna™ Lowest Recharge Burden Comparison Memo (MAT-2210739). 2023.
- Nanivadekar A, Falowski S, Benison A. Utilizing interoperative neuromonitoring to program multiple areas of BurstDR SCS for treatment of chronic pain. Neuromodulation. 2022;25(5):S86. https://www.neuromodulationjournal.org/article/S1094-7159(22)00399-3/abstract
- Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56-66. https://www.neuromodulationjournal.org/article/S1094-7159(21)02168-1/fulltext